The Role of AI in Powering Digital Protocols
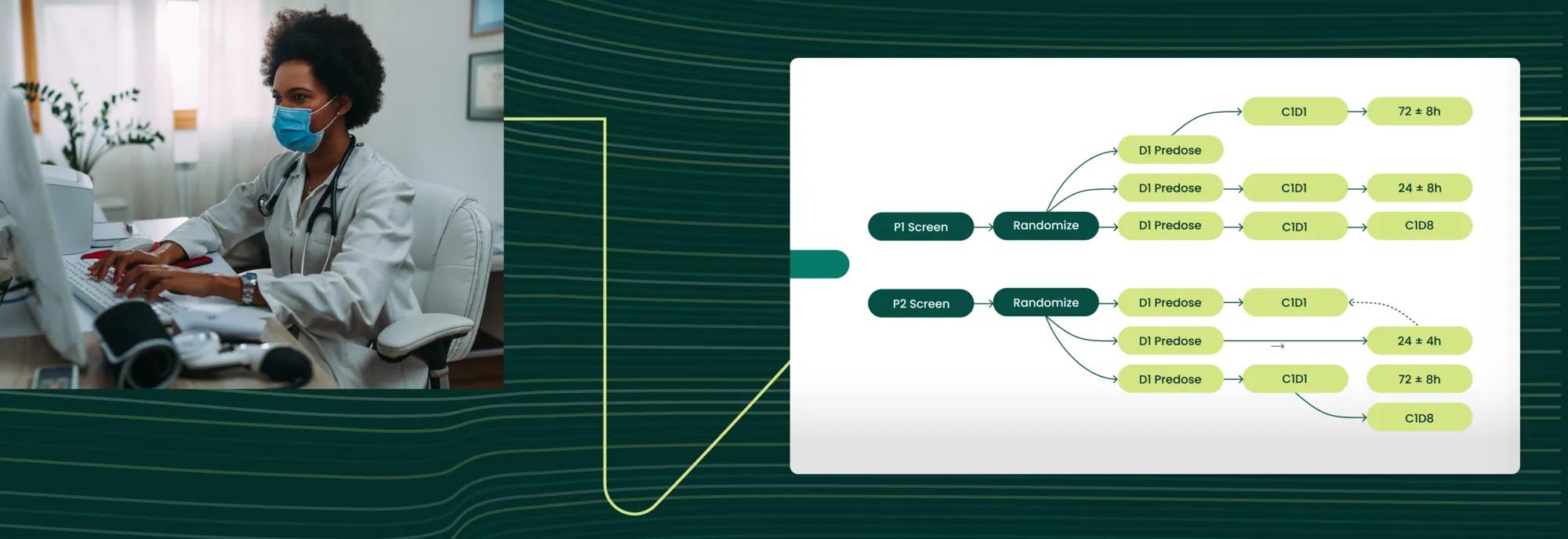
Pharmaceutical companies face mounting pressure to streamline trial processes, reduce costs, and accelerate drug development. Verily is addressing these challenges through innovative applications of artificial intelligence (AI) in protocol digitization.
The challenge of protocol interpretation
For many clinical trials, the process of translating a trial protocol into actionable steps across various systems is both labor-intensive and time-consuming. Each study requires data management teams to configure the study protocol into Electronic Data Capture (EDC) systems, site operations teams to input workflows and financials into their site CTMS systems, and sponsor teams to do the same for sponsor CTMS systems.
Configuring eCRFs in the EDC system alone can take 3-4 months for complex studies. This manual interpretation not only delays study startup, but also introduces the risk of inconsistencies across different systems—especially when the protocol is amended.
Verily’s AI-powered solution: Digital Protocols
Verily Viewpoint Digital Protocols serves as a comprehensive blueprint for study technologies, leveraging an opinionated data model to capture the nuances of trial design, such as complex branching, anchoring, cycle iterations, and unplanned visits.
Verily’s innovative solution combines the power of AI with clinical experts to transform static PDF protocols into our dynamic, digital data model that drives clinical operations.
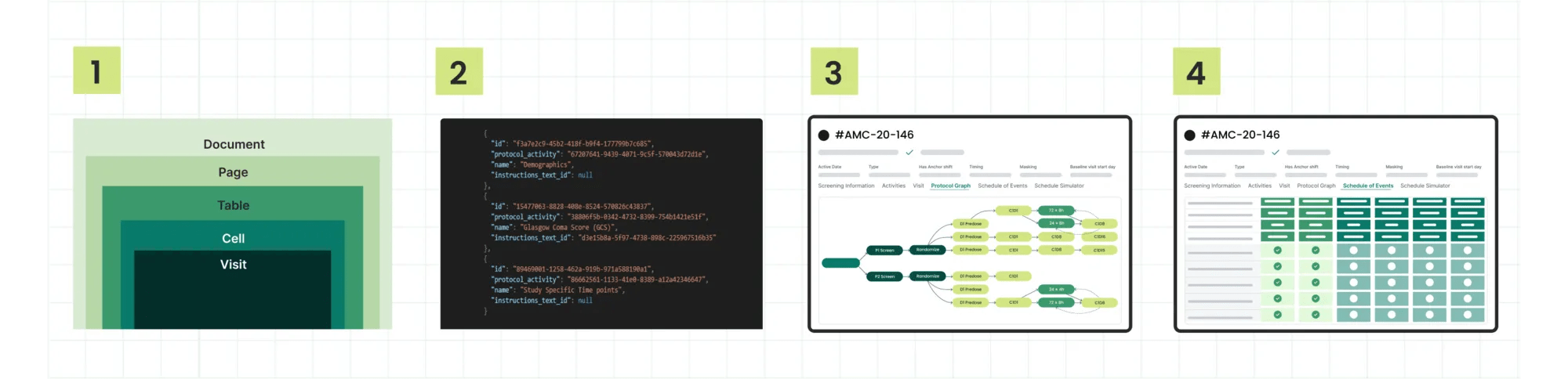
Process for creating Digital Protocols:
- AI Pre-processing: Advanced language learning models (LLMs) create a digital representation from a PDF study protocol.
- Structured Output: The AI solution outputs a JSON formatted digital protocol.
- Human Quality Assurance: Our team of clinical experts manually reviews the pre-processed configuration for quality assurance.
- System Integration: The digital protocol is pushed across downstream study systems (including Site CTMS).
This approach aligns with industry trends which are pushing trials towards common protocol data models. ICH M11 guidelines from the European Medicines Agency are expected to be finalized this year, establishing common protocol structures. In parallel, the Transcelerate Digital Data Flow Initiative has been a core component in establishing a common data model (CDISC’s USDM) for use in digital protocols for clinical studies.
Fine-tuning LLMs to automate the digitization workflow
Verily data scientists continue to research and implement AI into the Digital Protocol solution to improve digitization speed and effectiveness. A recent paper published in the Proceedings of Machine Learning Research demonstrates this work. Verily scientists investigated if labels generated by an LLM can effectively fine-tune another LLM for the complex task of identifying Schedule of Event (SoE) tables in clinical trial protocols.
The study found that LLM-generated labels achieve performance levels approaching those obtained with human-generated labels. By implementing a filtering mechanism, the team improved the quality of LLM-generated labels and demonstrated the potential of a hybrid labeling strategy combining human expertise with AI.
This research has the potential to not only reduce the burden of expert annotation but also pave the way for extending similar principles to other aspects of digital protocols beyond table detection.
Benefits & use cases for sponsors
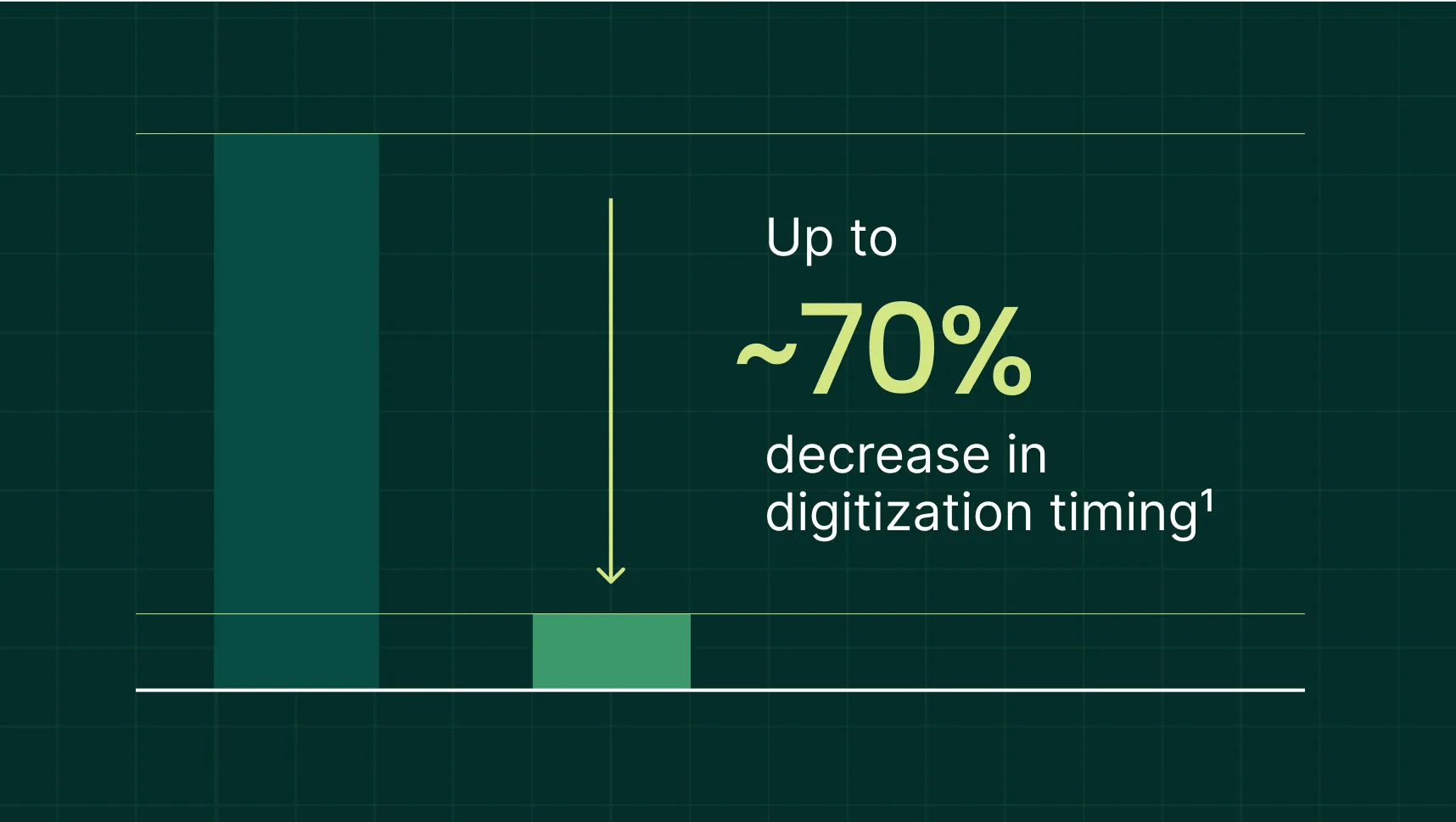
Our AI-driven approach has produced impressive results—including an up to 70% decrease in digitization time1. This translates to faster study startup timelines, reduced costs associated with manual system configuration, and increased scalability—enabling sponsors to manage more trials with existing resources.
And we’re continuing to develop and explore applications that can benefit sponsors, such as:
- Maintaining synchronization across all systems used in a study
- Driving increased connections between site and sponsor workflows
- Powering patient matching to trials
- Creating repositories of protocols to leverage for future trial design
- Automating clinical trial documentation, pricing, and eCRF creation
Partnering for success
Verily is dedicated to innovating and exploring new ways to leverage AI in clinical research. We’re committed to help build a future where clinical trials are faster, more accurate, and more accessible for everyone.
By partnering with Verily to leverage AI-powered Digital Protocols, pharmaceutical companies can more effectively navigate the challenges of clinical trials, ultimately bringing life-changing therapies to patients faster and more efficiently.
Reach out to start the discussion about how you can partner with Verily on your journey to digitize your clinical operations workflows, or learn more about Digital Protocols:
- Watch our webinar with Verily experts to learn how Digital Protocols are unlocking efficiencies to accelerate clinical trials
- Download the solution sheet: Optimize clinical trial operations with a dynamic, digital study blueprint
- Watch a short clip on Digital Protocols
1 Results from an individual Viewpoint Site CTMS client for amended protocols. New protocols saw a 60% decrease in digitization time.


